
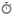
این تکنیک کاربردهای وسیعی در تشخیص بالینی و تحقیقات دارد. در این روش میتوان یک سکانس خاص را در DNAی هدف، جستجو و شناسایی کرد و همچنین مقدار آن را در نمونهای مورد مطالعه اندازه گیری نمود. در این تکنیک نیز اصول کلی PCR حاکم است ولی نکته حائز اهمیت این است که پس از تکثیر DNA در هر سیکل، غلظت دقیق آن اندازه گیری میشود. سایر نامهای این تکنیک عبارتند از quantitative real time polymerase chain reaction و kinetic polymerase chain reaction. این تکنیک گاهی به اشتباه RT-PCR نامیده میشود که مخفف روش Reverse Transcription PCR است. ولی امروزه در دنیای تجارت و حتی مراکز تحقیقاتی بصورت یک اشتباه مصطلح در آمده است. گاهی روش Real-time PCR با رونویسی معکوس(reverse transcription) ادغام میگردد تا مقدار mRNA (messenger RNA) در سلولها و بافتها اندازه گیری شود. این تکنیک جدید، Real-time reverse-transcription PCR نامیده میشود و با علامتهای اختصاری qRT-PCR، RRT-PCRو یا RT-rt PCR مشخص میگردد.
در تکنیک Real-time PCR، برای تعیین غلظت DNA از رنگهای فلورسانس و یا شاخص های الیگونوکلئوتیدی فلورسانس استفاده میشود.
تعیین غلظت DNA با استفاده از رنگهای فلورسانس
در این تکنیک از رنگهایی استفاده میشود که پس از اتصال به ساختار دو رشته ای DNA (double-stranded:ds)، نور فلورسانس منتشر میکنند. بنابر این در طی واکنش PCR، به موازات افزایش غلظت DNA، میزان فلورسانس در محلول افزایش می یابد. با اندازه گیری شدت نور فلورسانس در انتهای هر سیکل، نهایتا یک منحنی بدست می آید. سپس با استفاده از نمودارهای استاندارد، غلظت DNAی هدف در نمونه مورد مطالعه محاسبه میشود. لازم به ذکر است که رنگهایی نظیر SYBR Green به تمام انواع DNAی دو رشته ای، ازجمله محصولات غیر اختصاصی PCR و حتی دیمرهای پرایمر (Primer dimers) نیز متصل میشوند و این موضوع موجب کاهش اختصاصی بودن (Specificity) در نتایج حاصل میشود.
تعیین غلظت DNA با استفاده از شاخص های الیگونوکلئوتیدی فلورسانس
در تکنیک Real-time PCR، دقیقترین و قابل اعتمادترین نتایج با استفاده از شاخص های گزارشگر فلورسانس بدست می آید که البته هزینه زیادی نیز در بردارد. در این روش از شاخصهای DNA یا RNA ی اختصاصی (sequence-specific RNA or DNA-based probe) استفاده میشود و در نتیجه در DNAی هدف، جستجو و تعیین غلظت برای سکانسهای خاص امکانپذیر میگردد. بنابر این حتی در حضور سایر انواع DNA، نتایج حاصل از نظر اختصاصی بودن، در حد بالائی است. در این شرایط، چنانچه از شاخصهای اختصاصی با رنگهای مختلف استفاده شود، حتی میتوان ژنهای متعددی را در یک واکنش PCR، مورد بررسی قرار داد.
کاربردهای Real-time PCR:
1. تعیین تعداد کپی از هر ژن: Wu, 2007; ABI TaqMan® Gene Copy Number Assays; Protocol for 7900HT
2. سنجش میزان بیان ژن:Giulietti, 2001 و Leung, 2005
3. بررسی صحت نتایج در آرایه ها: Rajeevan, 2001 و Verification of Array Results Page by Pfaffl
4. مطالعات ایمنی زیستی و پایداری ژنتیک: Lovatt, 2002
5. بررسی میزان اثر بخشی داروها و مانیتورینگ داروها: Leruez-Ville, 2004; Brennan, 2003; Burger, 2003; Kogure, 2004
6. انجام تکنیک Real-Time Immuno-PCR (IPCR): Adler, 2003; Barletta, 2004; Lind & Kubista, 2005
7. رسوب کروماتین با استفاده از اتصال آنتی ژن-آنتی بادی: Braveman, 2004; Sandoval, 2004; Wang, 2004; Iype, 2005; Potratz, 2005; Puppo, 2005
8. سنجش ویروسها: Niesters, 2001; Mengelle, 2003; Espy, 2006
9. شناسائی عوامل پاتوژن شامل:
شناسائی CMV: Kearns, 2001a; Kearns, 2001b; Kearns, 2002; Mengelle, 2003
تشخیص سریع آلودگی به مننگوکوک: Bryant, 2004
بررسی حساسیت استرپتوکوکوس پنومونیا به پنی سیلین : Kearns, 2002
شناسائی مایکوباکتریوم توبرکولوزیس و گونه های مقاوم: Kraus, 2001; Torres, 2003; Cleary, 2003; Hazbon, 2004
پاتوژن های میکروبی در آبهای محیطی: Foulds, 2002; Guy, 2003
10. اندازه گیری میزان آسیب به DNA: Dietmaier, 2001
11. سنجش میزان تماس با اشعه رادیواکتیو: Blakely, 2001; Blakely, 2002; Grace, 2002; Grace, 2003
12. بررسی فرآیندهای زیستی در سلولهای زنده: Tung, 2000; Bremer, 2002
13. مطالعه DNAی میتوکندریائی: He, 2002; Liu, 2003; Alonso, 2004
14. شناسائی متیلاسیون: Trinh, 2001; Cottrell, 2004; Lehmann & Kreipe, 2004; Thomassin, 2004
15. تشخیص غیرفعال شدن ژنها در کروموزوم X: Hartshorn, 2002; van Dijk, 2002
16. تعیین همسانی در لوکوسهای HLA: Zhou, 2004
17. پیگیری نتایج پیوند عضو: Sabek, 2002; Gibbs, 2003
18. پیگیری نتایج، پس از پیوند سلولهای بنیادی خونساز: Elmaagacli, 2002; Alizadeh, 2002; Thiede, 2004; Harries, 2004
19. پیگیری عوارض باقیمانده ناشی از بیماری، پس از پیوند سلولهای بنیادی خونساز: Elmaagacli, 2002; Cilloni, 2002; Sarris, 2002; Gabert, 2003; Van der Velden, 2003
20. تعیین مقدار ژن و زیگوسیتی: (Bubner, 2004; Barrois, 2004; Chen, 2006; Szilagyi, 2006
21. ژنوتایپینگ و تشخیص انواع موتاسیونها شامل الحاق، حذف و موتاسیون نقطه ای: von Ahsen 2000; Donohoe, 2000; Lyon, 2001; Waterfall & Cobb, 2002; Bennett, 2003; Wittwer, 2003; Zhou, 2005; Palais, 2005; Chou, 2005، ; Mhlanga, 2001; Solinas, 2001; Song, 2002; Gupta, 2004; reviewed in Lareu, 2004، Kutyavin, 2000; Letertre, 2003; Johnson, 2004; Ugozzoli, 2004، Tapp, 2000
22. بررسی انواع تریزومی: Zimmermann, 2002
23. بررسی ژنوتیپهای حاصل از حذف جزئی یا microdeletion: Laurendeau, 1999; Kariyazono, 2001; Covault, 2003; Coupry, 2004
24. هاپلوتایپینگ: Von Ahsen, 2004; Pont-Kingdon & Lyon, 2005
25. بررسی کمی میکروساتلیت ها: Ginzinger, 2000
26. تشخیص قبل از تولد و تعیین جنسیت با استفاده از سلولهای جدا شده از خون مادرHahn, 2000; Bischoff, 2002; Bischoff, 2003 یا DNAی جنینی در جریان خون مادر Bischoff, 2002; Hwa, 2004
27. تشخیص قبل از تولد برای اختلالات هموگلوبین: Kanavakis, 1997; Vrettou, 2003; Vrettou, 2004
28. تشخیص سرطان در حین عمل جراحی : Raja, 2002
29 LATE-PCR که یک نوع real-time PCR جدید است و بررسی های کمی را در مقادیر جزئی از نمونه های بیولوژیک، امکانپذیر میسازد و بتریج کاربردهای وسیعی در تشخیص بالینی، دفاع بیولوژیک، پزشکی قانونی و تعیین سکانس DNA می یابد: Sanchez, 2004
منابع اینترنتی برای مطالعه بیشتر در زمینه Real-Time PCR
1st International qPCR Symposium & Application Workshop, qPCR 2009
ABgene Dual Labeled Probe Design Guide
ABI TaqMan Human Endogenous Control Plate
ABI User Bulletins ABI-PRISM 7700 Application Notes 7900HT 7000 Compendium
AlleleID Pathogen Detection Primer & Probe Design Tool by Premier Biosoft International
Ambion TechNotes on Real-Time PCR
Applied Biosystems Sequence Detection Systems
Automated PubMed Search for Real-Time PCR
Available Real-Time PCR Platforms BioCompare
BestKeeper© for determination of stable housekeeping genes, Download
برنامه های آموزشیBiocompare
BioInformatics in Real-Time PCR
BioTechniques Molecular Biology Forums: Real-Time qPCR
CAmpER – Real-time PCR Analysis Software
DesignMyProbe at Sigma-Aldrich
Essentials of Real-Time PCR Lecture by Man Bock Gu
Five Questions on qPCR & How It Works, The Scientist
Fluidigm, high-throughput qPCR, CNV, genotyping
Frequently Asked Questions, Real-Time PCR Primers
Full qPCR Protocol (Nolan, Hands & Bustin, Nature Protocols, 2006), PDF
Gene Quantification Page by Michael W Pfaffl & Directory Page
geNORM (Vandesompele, 2002) NormFinder (Andersen, 2004) qBasePlus, Hellemans, 2007
Idaho Technology LightScanner & FilmArray
Invitrogene Molecular Probes Handbook
Lab-on-a-Chip Technology: Biomolecular Separation and Analysis
Lab-on-a-Chip Technology: Fabrication and Microfluidics
LNA Primers, Exiqon OligoDesign
MIQE: Minimum Information for Publication of qPCR Experiments (Checklist: XLS, PDF) – Bustin, 2009
Open Access Real-time PCR Papers
PCR Troubleshooting: The Essential Guide
Peirson, 2003 (DART-PCR), Download
PrimerDesign InVitroGene: Custom Primers-OligoPerfect™ Designer
Primer-Probe and Beacon Design Program & Demo by Premier
QiaGen Handbooks on SYBR Green Detection Systems and RT-PCR
Q-PCR Training @ TATAA BioCenter
Quantitative PCR Gene Expression Profiling by MultiD – Tutorials
Quantitative PCR Primer Database – QPPD, NCI
Real Time PCR & Quantitation Lecture by Ian MacKay
Real Time PCR Special Issue (Dec 2001, Vol.25, Issue 4) of METHODS Journal
Real-Time PCR Handbook, University of Illinois at Chicago
Real-Time PCR in Infectious Diseases (PPT) & (PDF) by Theo Sloots
Real-Time PCR in Microbiology: From Diagnosis to Characterization
Real-Time PCR in Microbiology: From Diagnosis to Characterization
Real-Time PCR Seminar (NIEHS) & Review by Nigel Walker
Real-Time PCR Tutorial, South Carolina University
Real-Time PCR: Current Technology and Applications
Real-Time PCR: Current Technology and Applications
Real-Time PCR: Short Course, University of Texas
Real-Time PCR: Understanding CT
9REST© for Relative Expression Software Tool, REST-2008 / Corbett
Review of real-time PCR in mRNA Quantitation, Wong & Medrano, 2005
Roche LightCycler Literature and Technical Notes
Roche LightCycler One-Step RT-PCR Kit
Setting Baselines and Thresholds
Sigma-Aldrich qPCR Technical Guide
SNP500 Cancer Validated TaqMan Allelic Discrimination Assays
SNP500Cancer Validated Allelic Discrimination Assay List, including TaqMan Protocols
Statistics and Gene Expression Analysis
Stratagene Multiplex qPCR System & qPCR Guide
Stratagene Mx3000™ Multiplex Quantitative PCR System
STRATAGENE Online qPCR Training
TaqMan Human Endogenous Control Plate
TaqMan Human Endogenous Control Plate
TATAA Biocenter Endogenous Control Gene Panel
TATAA Biocenter Open Courses in Quantitative PCR
Tools and Technologies for Real-Time PCR & Fast PCR, text
Tools at TATAA BioCenter & GenEx
Transcript of a Webcast on Real-Time PCR Applications, Bio.Com
Troubleshooting & Optimization Guide, Thermo Scientific
Workshops and Courses by Stephen Bustin
کتابها
(Real-Time PCR (Dorak MT
(A-Z of Quantitative PCR (Bustin S
(Rapid Cycle Real-Time PCR-Methods and Applications (Wittwer Hahn, Kaul
(Real-Time PCR: An Essential Guide (Edwards, Logan, Saunders
(Real-Time PCR: Current Technology and Applications (Logan, Edwards, Saunders
(Real-time PCR in Microbiology (MacKay IM
Real-time PCR
From: Wikipedia, the free encyclopedia

 From: Wikipedia, the free encyclopedia
From: Wikipedia, the free encyclopedia
For reverse transcription polymerase chain reaction (RT-PCR), see reverse transcription polymerase chain reaction.
SYBR Green fluorescence chart for five samples, each having three replicates, which is a result of quantitative PCR (qPCR).
Melting curve for five samples, three replicates each, which is a result of melting temperature analysis of quantitative PCR results (qPCR).
A real-time polymerase chain reaction is a laboratory technique of molecular biology based on the polymerase chain reaction (PCR), which is used to amplify and simultaneously detect or quantify a targeted DNA molecule.
The procedure follows the general principle of polymerase chain reaction; its key feature is that the amplified DNA is detected as the reaction progresses in “real time”. This is a new approach compared to standard PCR, where the product of the reaction is detected at its end. Two common methods for the detection of products in quantitative PCR are: (1) non-specific fluorescent dyes that intercalate with any double-stranded DNA, and (2) sequence-specific DNA probes consisting of oligonucleotides that are labelled with a fluorescent reporter which permits detection only after hybridization of the probe with its complementary sequence to quantify messenger RNA (mRNA) and non-coding RNA in cells or tissues.
The MIQE guidelines propose that the abbreviation qPCR be used for quantitative real-time PCR and that RT-qPCR be used for reverse transcription–qPCR [1]. The acronym “RT-PCR” commonly denotes reverse transcription polymerase chain reaction and not real-time PCR, but not all authors adhere to this convention.[1]
Background
 From: Wikipedia, the free encyclopedia
From: Wikipedia, the free encyclopedia
Real time quantitative PCR uses fluorophores in order to detect levels of gene expression.
Cells in all organisms regulate gene expression by turnover of gene transcripts (messenger RNA, abbreviated to mRNA): The amount of an expressed gene in a cell can be measured by the number of copies of an mRNA transcript of that gene present in a sample. In order to robustly detect and quantify gene expression from small amounts of RNA, amplification of the gene transcript is necessary. The polymerase chain reaction (PCR) is a common method for amplifying DNA; for mRNA-based PCR the RNA sample is first reverse-transcribed to cDNA with reverse transcriptase.
In order to amplify small amounts of DNA, the same methodology is used as in conventional PCR using a DNA template, at least one pair of specific primers, deoxyribonucleotides, a suitable buffer solution and a thermo-stable DNA polymerase. A substance marked with a fluorophore is added to this mixture in a thermal cycler that contains sensors for measuring the fluorescence of the flurophore after it has been excited at the required wavelength allowing the generation rate to be measured for one or more specific products. This allows the rate of generation of the amplified product to be measured at each PCR cycle. The data thus generated can be analysed by computer software to calculate relative gene expression (or mRNA copy number) in several samples. Quantitative PCR can also be applied to the detection and quantification of DNA in samples to determine the presence and abundance of a particular DNA sequence in these samples.[2] This measurement is made after each amplification cycle, and this is the reason why this method is called real time PCR (that is, immediate or simultaneous PCR). In the case of RNA quantitation, the template is complementary DNA (cDNA), which is obtained by reverse transcription of ribonucleic acid (RNA). In this instance the technique used is quantitative RT-PCR or Q-RT-PCR.
Quantitative PCR and DNA microarray are modern methodologies for studying gene expression. Older methods were used to measure mRNA abundance: Differential display, RNase protection assay and Northern blot. Northern blotting is often used to estimate the expression level of a gene by visualizing the abundance of its mRNA transcript in a sample. In this method, purified RNA is separated by agarose gel electrophoresis, transferred to a solid matrix (such as a nylon membrane), and probed with a specific DNA or RNA probe that is complementary to the gene of interest. Although this technique is still used to assess gene expression, it requires relatively large amounts of RNA and provides only qualitative or semi quantitative information of mRNA levels.[3] Estimation errors arising from variations in the quantification method can be the result of DNA integrity, enzyme efficiency and many other factors. For this reason a number of standardization systems have been developed. Some have been developed for quantifying total gene expression, but the most common are aimed at quantifying the specific gene being studied in relation to another gene called a normalizing gene, which is selected for its almost constant level of expression. These genes are often selected from housekeeping genes as their functions related to basic cellular survival normally implie constitutive gene expression.[4][5] This enables researchers to report a ratio for the expression of the genes of interest divided by the expression of the selected normalizer, thereby allowing comparison of the former without actually knowing its absolute level of expression.
The most commonly used normalizing genes are those that code for the following molecules: tubulin, glyceraldehyde-3-phosphate dehydrogenase, albumin, cyclophilin, and ribosomal RNAs.[3]
Basic principles
From: Wikipedia, the free encyclopedia
Quantitative PCR is carried out in a thermal cycler with the capacity to illuminate each sample with a beam of light of a specified wavelength and detect the fluorescence emitted by the excited fluorophore. The thermal cycler is also able to rapidly heat and chill samples, thereby taking advantage of the physicochemical properties of the nucleic acids and DNA polymerase.
The PCR process generally consists of a series of temperature changes that are repeated 25 – 40 times. These cycles normally consist of three stages: the first, at around 95 °C, allows the separation of the nucleic acid’s double chain; the second, at a temperature of around 50-60 °C, allows the binding of the primers with the DNA template;[6] the third, at between 68 – 72 °C, facilitates the polymerization carried out by the DNA polymerase. Due to the small size of the fragments the last step is usually omitted in this type of PCR as the enzyme is able to increase their number during the change between the alignment stage and the denaturing stage. In addition, some thermal cyclers add another short temperature phase lasting only a few seconds to each cycle, with a temperature of, for example, 80 °C, in order to reduce the noise caused by the presence of primer dimers when a non-specific dye is used. The temperatures and the timings used for each cycle depend on a wide variety of parameters, such as: the enzyme used to synthesize the DNA, the concentration of divalent ions and deoxyribonucleotides (dNTPs) in the reaction and the bonding temperature of the primers.[7]
Classification
From: Wikipedia, the free encyclopedia
The type of quantitative PCR technique used depends on the DNA sequence in the samples, the technique can either use non-specific fluorochromes or hybridization probes.
Quantitative PCR with double-stranded DNA-binding dyes as reporters
A DNA-binding dye binds to all double-stranded (ds) DNA in PCR, causing fluorescence of the dye. An increase in DNA product during PCR therefore leads to an increase in fluorescence intensity and is measured at each cycle, thus allowing DNA concentrations to be quantified. However, dsDNA dyes such as SYBR Green will bind to all dsDNA PCR products, including nonspecific PCR products (such as Primer dimer). This can potentially interfere with, or prevent, accurate quantification of the intended target sequence. The SYBR Green is excited using blue light (λmax = 488 nm) and it emits green light (λmax = 522 nm).[8]
- The reaction is prepared as usual, with the addition of fluorescent dsDNA dye.
- The reaction is run in a quantitative PCR instrument, and after each cycle, the levels of fluorescence are measured with a detector; the dye only fluoresces when bound to the dsDNA (i.e., the PCR product). With reference to a standard dilution, the dsDNA concentration in the PCR can be determined.
This method has the advantage of only needing a pair of primers to carry out the amplification, which keeps costs down; however, it is only possible to amplify a product using a chain reaction.
Like other quantitative PCR methods, the values obtained do not have absolute units associated with them (i.e., mRNA copies/cell). As described above, a comparison of a measured DNA/RNA sample to a standard dilution will only give a fraction or ratio of the sample relative to the standard, allowing only relative comparisons between different tissues or experimental conditions. To ensure accuracy in the quantification, it is usually necessary to normalize expression of a target gene to a stably expressed gene (see below). This can correct possible differences in RNA quantity or quality across experimental samples.
Fluorescent reporter probe method
 From: Wikipedia, the free encyclopedia
From: Wikipedia, the free encyclopedia
(1) In intact probes, reporter fluorescence is quenched. (2) Probes and the complementary DNA strand are hybridized and reporter fluorescence is still quenched. (3) During PCR, the probe is degraded by the Taq polymerase and the fluorescent reporter released.
Fluorescent reporter probes detect only the DNA containing the probe sequence; therefore, use of the reporter probe significantly increases specificity, and enables quantification even in the presence of non-specific DNA amplification. Fluorescent probes can be used in multiplex assays—for detection of several genes in the same reaction—based on specific probes with different-coloured labels, provided that all targeted genes are amplified with similar efficiency. The specificity of fluorescent reporter probes also prevents interference of measurements caused by primer dimers, which are undesirable potential by-products in PCR. However, fluorescent reporter probes do not prevent the inhibitory effect of the primer dimers, which may depress accumulation of the desired products in the reaction.
The method relies on a DNA-based probe with a fluorescent reporter at one end and a quencher of fluorescence at the opposite end of the probe. The close proximity of the reporter to the quencher prevents detection of its fluorescence; breakdown of the probe by the 5′ to 3′ exonuclease activity of the Taq polymerase breaks the reporter-quencher proximity and thus allows unquenched emission of fluorescence, which can be detected after excitation with a laser. An increase in the product targeted by the reporter probe at each PCR cycle therefore causes a proportional increase in fluorescence due to the breakdown of the probe and release of the reporter.
- The PCR is prepared as usual (see PCR), and the reporter probe is added.
- As the reaction commences, during the annealing stage of the PCR both probe and primers anneal to the DNA target.
- Polymerisation of a new DNA strand is initiated from the primers, and once the polymerase reaches the probe, its 5′-3′-exonuclease degrades the probe, physically separating the fluorescent reporter from the quencher, resulting in an increase in fluorescence.
- Fluorescence is detected and measured in a real-time PCR machine, and its geometric increase corresponding to exponential increase of the product is used to determine the quantification cycle (Cq) in each reaction.
Fusion temperature analysis
From: Wikipedia, the free encyclopedia
Distinct fusion curves for a number of PCR products (showing distinct colours). Amplification reactions can be seen for a specific product (pink, blue) and others with a negative result (green, orange). The fusion peak indicated with an arrow shows the peak caused by primer dimers, which is different from the expected amplification product.[9]
Q-PCR permits the identification of specific, amplified DNA fragments using analysis of their melting temperature (also called Tm value, from melting temperature). The method used is usually PCR with double-stranded DNA-binding dyes as reporters and the dye used is usually SYBR Green. The DNA melting temperature is specific to the amplified fragment. The results of this technique are obtained by comparing the dissociation curves of the analysed DNA samples.[10]
Unlike conventional PCR, this method avoids the previous use of electrophoresis techniques to demonstrate the results of all the samples. This is because, despite being a kinetic technique, quantitative PCR is usually evaluated at a distinct end point. The technique therefore usually provides more rapid results and / or uses fewer reactants than electrophoresis. If subsequent electrophoresis is required it is only necessary to test those samples that real time PCR has shown to be doubtful and / or to ratify the results for samples that have tested positive for a specific determinant.
Quantification of gene expression
Quantifying gene expression by traditional DNA detection methods is unreliable. Detection of mRNA on a Northern blot or PCR products on a gel or Southern blot does not allow precise quantification.[11] For example, over the 20-40 cycles of a typical PCR, the amount of DNA product reaches a plateau that is not directly correlated with the amount of target DNA in the initial PCR.[citation needed]
Quantitative PCR can be used to quantify nucleic acids by two common methods: relative quantification and absolute quantification.[12] Absolute quantification gives the exact number of target DNA molecules by comparison with DNA standards using a calibration curve. It is therefore essential that the PCR of the sample and the standard have the same amplification efficiency. Relative quantification is based on internal reference genes to determine fold-differences in expression of the target gene. The quantification is expressed as the change in expression levels of mRNA interpreted as complementary DNA (cDNA, generated by reverse transcription of mRNA). Relative quantification is easier to carry out as it does not require a calibration curve as the amount of the studied gene is compared to the amount of a control housekeeping gene.
As the units used to express the results of relative quantification are unimportant the results can be compared across a number of different RT-Q-PCR. The reason for using one or more housekeeping genes is to correct non-specific variation, such as the differences in the quantity and quality of RNA used, which can affect the efficiency of reverse transcription and therefore that of the whole PCR process. However, the most crucial aspect of the process is that the reference gene must be stable.[13]
The selection of these reference genes was traditionally carried out in molecular biology using qualitative or semi-quantitative studies such as the visual examination of RNA gels, Northern blot densitometry or semi-quantitative PCR (PCR mimics). Now, in the genome era, it is possible to carry out a more detailed estimate for many organisms using DNA microarrays.[14] However, research has shown that amplification of the majority of reference genes used in quantifying the expression of mRNA varies according to experimental conditions.[15][16][17] It is therefore necessary to carry out an initial statistically sound methodological study in order to select the most suitable reference gene.
A number of statistical algorithms have been developed that can detect which gene or genes are most suitable for use under given conditions. Those like geNORM or BestKeeper can compare pairs or geometric means for a matrix of different reference genes and tissues.[18][19]
Modeling
Unlike end point PCR (conventional PCR) real time PCR allows quantification of the desired product at any point in the amplification process by measuring fluorescence (in reality, measurement is made of its level over a given threshold). A commonly employed method of DNA quantification by quantitative PCR relies on plotting fluorescence against the number of cycles on a logarithmic scale. A threshold for detection of DNA-based fluorescence is set slightly above background. The number of cycles at which the fluorescence exceeds the threshold is called the threshold cycle (Ct) or, according to the MIQE guidelines, quantification cycle (Cq).[20]
During the exponential amplification phase, the quantity of the target DNA template (amplicon) doubles every cycle. For example, a DNA sample whose Cq precedes that of another sample by 3 cycles contained 23 = 8 times more template. However, the efficiency of amplification is often variable among primers and templates. Therefore, the efficiency of a primer-template combination is assessed in a titration experiment with serial dilutions of DNA template to create a standard curve of the change in Cq with each dilution. The slope of the linear regression is then used to determine the efficiency of amplification, which is 100% if a dilution of 1:2 results in a Cq difference of 1. The cycle threshold method makes several assumptions of reaction mechanism and has a reliance on data from low signal-to-noise regions of the amplification profile that can introduce substantial variance during the data analysis.[21]
To quantify gene expression, the Cq for an RNA or DNA from the gene of interest is subtracted from the Cq of RNA/DNA from a housekeeping gene in the same sample to normalize for variation in the amount and quality of RNA between different samples. This normalization procedure is commonly called the ΔCt-method[22] and permits comparison of expression of a gene of interest among different samples. However, for such comparison, expression of the normalizing reference gene needs to be very similar across all the samples. Choosing a reference gene fulfilling this criterion is therefore of high importance, and often challenging, because only very few genes show equal levels of expression across a range of different conditions or tissues.[23][24] Although cycle threshold analysis is integrated with many commercial software systems, there are more accurate and reliable methods of analysing amplification profile data that should be considered in cases where reproducibility is a concern.[21]
Mechanism-based qPCR quantification methods have also been suggested, and have the advantage that they do not require a standard curve for quantification. Methods such as MAK2[25] have been shown to have equal or better quantitative performance to standard curve methods. These mechanism-based methods use knowledge about the polymerase amplification process to generate estimates of the original sample concentration. An extension of this approach includes an accurate model of the entire PCR reaction profile, which allows for the use of high signal-to-noise data and the ability to validate data quality prior to analysis.[21]
Applications
There are numerous applications for quantitative polymerase chain reaction in the laboratory. It is commonly used for both diagnostic and basic research. Uses of the technique in industry include the quantification of microbial load in foods or on vegetable matter, the detection of GMOs (Genetically modified organisms) and the quantification and genotyping of human viral pathogens.
Diagnostic uses
Diagnostic quantitative PCR is applied to rapidly detect nucleic acids that are diagnostic of, for example, infectious diseases, cancer and genetic abnormalities. The introduction of quantitative PCR assays to the clinical microbiology laboratory has significantly improved the diagnosis of infectious diseases,[26] and is deployed as a tool to detect newly emerging diseases, such as new strains of flu, in diagnostic tests.[27]
Microbiological uses
Quantitative PCR is also used by microbiologists working in the fields of food safety, food spoilage and fermentation and for the microbial risk assessment of water quality (drinking and recreational waters) and in public health protection.[28]
The antibacterial assay Virtual Colony Count[29] utilizes a data quantification technique called Quantitative Growth Kinetics (QGK) that is mathematically identical to QPCR, except bacterial cells, rather than copies of a PCR product, increase exponentially. The QGK equivalent of the threshold cycle is referred to as the “threshold time”.
Uses in research
In research settings, quantitative PCR is mainly used to provide quantitative measurements of gene transcription. The technology may be used in determining how the genetic expression of a particular gene changes over time, such as in the response of tissue and cell cultures to an administration of a pharmacological agent, progression of cell differentiation, or in response to changes in environmental conditions. It is also used for the determination of zygosity of transgenic animals used in research.
Detection of phytopathogens
The agricultural industry is constantly striving to produce plant propagules or seedlings that are free of pathogens in order to prevent economic losses and safeguard health. Systems have been developed that allow detection of small amounts of the DNA of Phytophthora ramorum, an oomycete that kills Oaks and other species, mixed in with the DNA of the host plant. Discrimination between the DNA of the pathogen and the plant is based on the amplification of ITS sequences, spacers located in ribosomal RNA gene’s coding area, which are characteristic for each taxon.[30] Field-based versions of this technique have also been developed for identifying the same pathogen.[31]
Detection of genetically modified organisms
qPCR using reverse transcription (RT-qPCR) can be used to detect GMOs given its sensitivity and dynamic range in detecting DNA. Alternatives such as DNA or protein analysis are usually less sensitive. Specific primers are used that amplify not the transgene but the promoter, terminator or even intermediate sequences used during the process of engineering the vector. As the process of creating a transgenic plant normally leads to the insertion of more than one copy of the transgene its quantity is also commonly assessed. This is often carried out by relative quantification using a control gene from the treated species that is only present as a single copy.[32][33]
Clinical quantification and genotyping
Viruses can be present in humans due to direct infection or co-infections. This makes diagnosis difficult using classical techniques and can result in an incorrect prognosis and treatment. The use of qPCR allows both the quantification and genotyping (characterization of the strain, carried out using melting curves) of a virus such as the Hepatitis B virus.[34] The degree of infection, quantified as the copies of the viral genome per unit of the patient’s tissue, is relevant in many cases; for example, the probability that the type 1 herpes simplex virus reactivates is related to the number of infected neurons in the ganglia.[35] This quantification is carried out either with reverse transcription or without it, as occurs if the virus becomes integrated in the human genome at any point in its cycle, such as happens in the case of HPV (human papillomavirus), where some of its variants are associated with the appearance of cervical cancer.[36]
References
From: Wikipedia, the free encyclopedia
- edited by Julie Logan, Kirstin Edwards, and Nick Saunders. (2009). Logan J, Edwards K, Saunders N, ed. Real-Time PCR: Current Technology and Applications. Caister Academic Press. ISBN978-1-904455-39-4.
- D. Watson; Baker, T. A.; Bell, S. P.; Gann, A.; Levine, M. et Losick, R (2004). Molecular Biology of the Gene (Fifth ed.). San Francisco: Benjamin Cummings. ISBN0-321-22368-3. Cite uses deprecated parameter |coauthors= (help)
- ^ Jump up to: a b Michael W. Pfaff, Ales Tichopad, Christian Prgomet and Tanja P. Neuvians (2005). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations Biotechnology Letters 26:509-515
- Pfaffl, MW; Horgan, GW; Dempfle, L (2002). “Relative Expression Software Tool (REST©) for group wise comparison and statistical analysis of relative expression results in real-time PCR”. Acids Res. 30: e36.
- Vandesompele, J; De Preter, K; Pattyn, F; Poppe, B; Van Roy, N; De Paepe, A; Speleman, F (2002). “Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes”. Biol. 3: 1–12.
- Rychlik W, Spencer WJ, Rhoads RE (1990). “Optimization of the annealing temperature for DNA amplification in vitro“. Nucl Acids Res 18 (21): 6409–6412. doi:1093/nar/18.21.6409. PMC332522. PMID 2243783.
- Joseph Sambrook and David W. Russel (2001). Molecular Cloning: A Laboratory Manual (3rd ed.). Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press. ISBN0-87969-576-5.
- Zipper et al. (2004). “Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications”. Nucleic Acids Res 32 (12): e103. doi:1093/nar/gnh101. PMC484200. PMID 15249599. CS1 maint: Explicit use of et al. (link)
- Ponchel F, Toomes C, Bransfield K, Leong F.T, Douglas S.H, Field S.L, Bell S.M, Combaret V, Puisieux A, Mighell A.J (2003). “Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions” (W). BMC Biotechnol 3: 18. doi:1186/1472-6750-3-18.
- Ririe K.M, Rasmussen R.P, Wittwer C.T. (1997). “Product Differentiation by Analysis of DNA Melting Curves during the Polymerase Chain Reaction” (PDF). Analytical Biochemistry 245 (2): 154–160. doi:1006/abio.1996.9916. PMID9056205.
- Bruce Gelerter. “PEMF For Treatment Of Corneal Disorders”.
- Dhanasekaran,T. Mark Doherty, John Kenneth and TB Trials Study Group. (Mar 2010). “Comparison of different standards for real-time PCR-based absolute quantification”. Immunol Methods. 354 (1–2): 34–9. doi:10.1016/j.jim.2010.01.004. PMID20109462.
- Brunner, AM; Yakovlev, IA; Strauss, SH (2004). “Validating internal controls for quantitative plant gene expression studies”. BMC Plant Biol 4: 14.
- Czechowski, T; Stitt, M; Altmann, T; Udvardi, MK; Scheible, WR (2005). “Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis”. Plant Physiol 139: 5–17. doi:1104/pp.105.063743.
- Thellin, O; Zorzi, W; Lakaye, B; De Borman, B; Coumans, B; Henne, G; Grisar, T; Igout, A; Heinen, E (1999). “Housekeeping genes as internal standards: use and limits”. J Biotechnol 75: 197–200.
- Radonic, A; Thulke, S; Mackay, IM; Landt, O; Siegert, W; Nitsche, A (2004). “Guideline for reference gene selection for quantitative real-time PCR”. Biochem Biophys Res Commun 313: 856–862. doi:1016/j.bbrc.2003.11.177.
- Dheda, K; Huggett, JF; Bustin, SA; Johnson, MA; Rook, G; Zumla, A (2004). “Validation of housekeeping genes for normalizing RNA expression in real-time PCR”. Biotechniques 37: 112–119.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes” Genome Biol 37: RESEARCH0034
- Pfaffl, MW; Tichopad, A; Prgomet, C; Neuvians, TP (2004). “Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations”. Biotechnol Lett 26: 509–515. doi:1023/b:bile.0000019559.84305.47.
- Stephen A. Bustin, Vladimir Benes, Jeremy A. Garson, Jan Hellemans, Jim Huggett, Mikael Kubista, Reinhold Mueller, Tania Nolan, Michael W. Pfaffl, Gregory L. Shipley, Jo Vandesompele, and Carl T. Wittwer. (Apr 2009). “The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments”. Clin Chem. 55 (4): :611–22. doi:1373/clinchem.2008.112797. PMID19246619.
- Carr, A. C.; Moore, S. D. (2012). Lucia, Alejandro, ed. “Robust Quantification of Polymerase Chain Reactions Using Global Fitting”. PLoS ONE 7 (5): e37640. doi:1371/journal.pone.0037640. PMC3365123. PMID 22701526. edit
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H (2006). “Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula”. J Mol Med 84 (11): 901–10. doi:1007/s00109-006-0097-6. PMID16972087.
- Nailis H, Coenye T, Van Nieuwerburgh F, Deforce D, Nelis HJ (2006). “Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR”. BMC Mol Biol. 7 (1): 25. doi:1186/1471-2199-7-25. PMC1557526. PMID 16889665.
- Nolan T, Hands RE, Bustin SA (2006). “Quantification of mRNA using real-time RT-PCR”. Protoc. 1 (3): 1559–1582. doi:10.1038/nprot.2006.236. PMID17406449.
- Boggy G, Woolf PJ (2010). Ravasi, Timothy, ed. “A Mechanistic Model of PCR for Accurate Quantification of Quantitative PCR Data”. PLOS One 5 (8): e12355. doi:1371/journal.pone.0012355. PMC2930010. PMID 20814578.
- Sails AD (2009). “Applications in Clinical Microbiology”. Real-Time PCR: Current Technology and Applications. Caister Academic Press. ISBN978-1-904455-39-4.
- FDA Authorizes Emergency Use of Influenza Medicines, Diagnostic Test in Response to Swine Flu Outbreak in Humans. FDA News, April 27, 2009.
- Filion, M (editor) (2012). Quantitative Real-time PCR in Applied Microbiology. Caister Academic Press. ISBN978-1-908230-01-0.
- Ericksen B, Wu Z, Lu W, Lehrer RI. (2005). “Antibacterial Activity and Specificity of the Six Human α-Defensins”. Antimicrob Agents Chemother. 49 (1): 269–75. doi:1128/AAC.49.1.269-275.2005. PMC538877. PMID 15616305.
- Baldwin, B.G. (1992). “Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: An example from the Compositaogy”. Molecular Phylogenetics and Evolution 1 (1): 3–16. doi:1016/1055-7903(92)90030-K. PMID1342921.
- Tomlinson, J. A.; Barker, I.; Boonham, N. (2007). “Faster, Simpler, More-Specific Methods for Improved Molecular Detection of Phytophthora ramorum in the Field”. Applied and Environmental Microbiology 73 (12): 4040–4047. doi:1128/AEM.00161-07. PMC1932743. PMID 17449689.
- Holst-jensen A, R{o}nning S.B, L{o}vseth A, Berdal K.G. (2003). “PCR technology for screening and quantification of genetically modified organisms (GMOs)” (PDF). Analytical and Bioanalytical Chemistry 375 (8): 985–993. doi:1007/s00216-003-1767-7.
- Brodmann P.D, Ilg E.C, Berthoud H, Herrmann A. (2002). “… -Time Quantitative Polymerase Chain Reaction Methods for Four Genetically Modified Maize Varieties …”. Journal of AOAC International 85 (3): 646–653. doi:5555/jaoi.2002.85.3.646. PMID12083257.
- Yeh S.H. Tsai C.Y. Kao J.H. Liu C.J. Kuo T.J. Lin M.W. Huang W.L. Lu S.F. Jih J. Chen D.S. Others (2004). “Quantification and genotyping of hepatitis B virus in a single reaction by real-time PCR and melting …”. Journal of Hepatology 41 (4): 659–666. doi:1016/j.jhep.2004.06.031. PMID15464248.
- Sawtell N.M. (1998). “The Probability of in Vivo Reactivation of Herpes Simplex Virus Type 1 Increases with the Number of Latently Infected Neurons in the Ganglia”. Journal of Virology 72 (8): 6888–6892. PMC109900. PMID 9658140.
- Peter M. Rosty C. Couturier J. Radvanyi F. Teshima H. Sastre-garau X. (2006). “MYC activation associated with the integration of HPV DNA at the MYC locus in genital tumours” (W). Oncogene 25 (44): 5985–5993. doi:1038/sj.onc.1209625. PMID16682952.
Bibliography
- Elyse; Houde, Alain (2002). “La PCR en temps réel: principes et applications” (PDF). Reviews in Biology and Biotechnology 2 (2): 2–11.
- Bustin, SA (2000). “Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays”. J Mol Endocrinol 25 (2): 169–93. doi:10.1677/jme.0.0250169. PMID 11013345.
- Higuchi, R.; Dollinger, G.; Walsh, P.S.; Griffith, R. (1992). “Simultaneous amplification and detection of specific DNA-sequences”. Bio-Technology 10 (4): 413–417. doi:10.1038/nbt0492-413.
- Holland, P.M.; Abramson, R.D.; Watson, R.; Gelfand, D.H. (1991). “Detection of specific polymerase chain reaction product by utilizing the 50 !30 exonuclease activity of Thermus aquaticus DNA polymerase”. Proc. Natl. Acad. Sci. USA 88 (16): 7276–7280. JSTOR 2357665.
- Kubista, M; Andrade, JM; Bengtsson, M; Forootan, A; Jonak, J; Lind, K; Sindelka, R; Sjoback, R; Sjogreen, B; Strombom, L; Stahlberg, A; Zoric, N (2006). “The real-time polymerase chain reaction”. Mol Aspects Med. 27 (2-3): 95–125. doi:10.1016/j.mam.2005.12.007. PMID 16460794.
- Higuchi, R.; Fockler, C.; Dollinger, G.; Watson, R. (1993). “Kinetic PCR: Real time monitoring of DNA amplification reactions”. Biotechnology 11: 1026–1030. doi:10.1038/nbt0993-1026.
- Filion, M. (2012). “Quantitative Real-time PCR in Applied Microbiology.” Caister Academic Press. ISBN 978-1-908230-01-0
- Wawrik, B; Paul, JH; Tabita, FR (2002). “Real-time PCR quantification of rbcL (ribulose-1,5-bisphosphate carboxylase/oxygenase) mRNA in diatoms and pelagophytes”. Appl. Environ. Microbiol 68: 3771–3779. doi:10.1128/aem.68.8.3771-3779.2002.
- Logan J, Edwards K, Saunders N (editors) (2009). Real-Time PCR: Current Technology and Applications. Caister Academic Press. ISBN 978-1-904455-39-4.
External links
- Beginners Guide to Real Time PCR by Primerdesign
- The Reference in Q-PCR Academic & Industrial Information Platform
- Real Time PCR Tutorial by Dr Margaret Hunt, University of South Carolina, September 5, 2006
- openwetware
- RefGenes Open Access online tool to identify tissue specific reference genes for RT-qPCR
- Realtime PCR user experiences
- Articles about Real Time Pcr
- Basics about qPCR


